Thermodynamic is a science which deals with heat and work and energy transfer. One of the thermodynamic properties which predict whether a process will occur spontaneously at constant temperature and pressure is known as Gibbs energy. Josiah Williard Gibbs proposed this property in 1876. It can be defined as the free energy of the system is the sum of its enthalpy plus the product of the temperature and entropy of the system.
Mathematical expression for Gibbs energy is defined as,
G = H – TS
Where,
G = Gibbs energy
H = enthalpy
T = temperature
S = entropy
Note:
For a system at constant temperature and pressure,
ΔG>0; the reaction is non-spontaneous
ΔG<0; the reaction is spontaneous
ΔG=0; the reaction is at equilibrium
Endergonic and Exergonic reactions
Endergonic reactions are the reactions having ΔG positive and required additional energy. Here the products will have more energy than the reactants. They are non-spontaneous.
Exergonic reactions are the reactions that have a negative ΔG. The reactants will have more energy than the products. They don’t require additional energy to proceed with the reaction. They are spontaneous.
How Enthalpy, Entropy, and Gibbs Free energy is related?
Enthalpy is a measure of how much heat is absorbed in a chemical reaction or we can say that it is the total heat content in the system.
∆H = HP – Hr (where p = products; r = reactants)
Entropy is a measure of the dispersal of energy or matter that takes place in a reaction or it is expressed as a disorder in a system.
Gibbs free energy can be defined by combining these two thermodynamic properties.
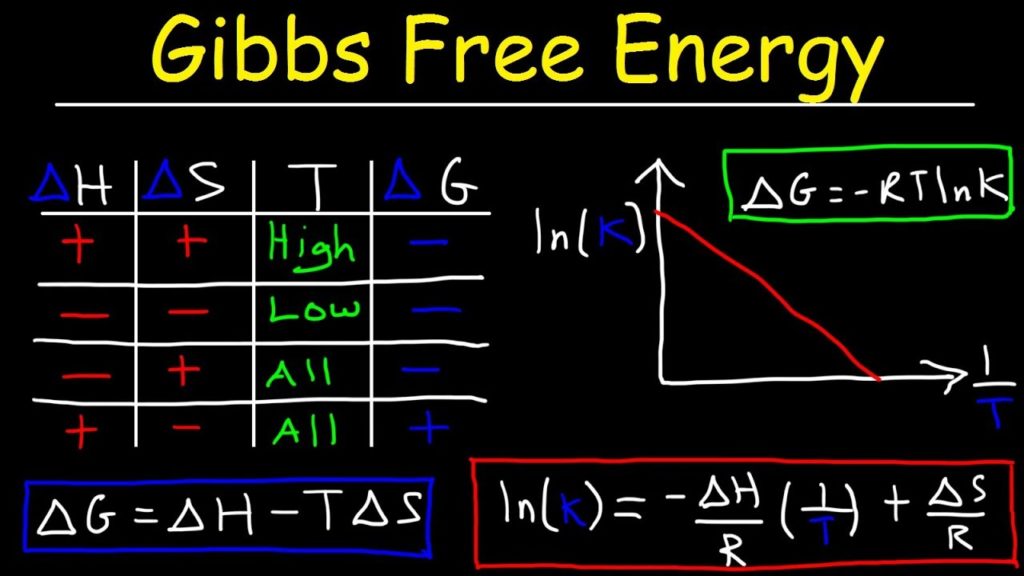
ΔG= ΔH – TΔS
- If ΔH is negative and ΔS is positive then, ΔG will be negative (over all temperature)
- If ΔH is negative and ΔS is negative then, ΔG will be negative (at high temperature)
- If ΔH is positive and ΔS is negative then, ΔG will be negative (at any temperature).
- If ΔH is positive and ΔS is positive then, ΔG will be negative (at low temperature)
Solubility Product
It is a heterogeneous equilibrium constant which is relevant for saturated solutions. Solubility product is the mathematical product of its dissolved ion concentration to the power of stoichiometric coefficients. It is also called an equilibrium constant.
An expression for Solubility Product
An equilibrium state will be there for saturated solutions between solid and its dissolved ions.
For example, we can consider an ionic compound A3B. we write the concentration of ions as
[A] and [B]
The solubility product or equilibrium constant is,
KSP = [A]3[B]
Gibbs Energy and Solubility Product
ΔG = ΔGo + RT InQ
Where Q is the reaction quotient
When equilibrium is attained, there is no further free energy change i.e. ΔG = 0 and Q becomes equal to equilibrium constant. Hence the above equation becomes.
ΔGo = –RT In K(eq)
An example is taken when calcium chloride dissolves in water, ΔH will be negative, ΔS will be positive, and results in large negative Gibbs energy and solubility.
Uses of Solubility Product
- The smaller the solubility product of a substance, the lower is its solubility.
- It can predict whether a precipitate will form when two solutions are mixed under specific conditions
Summary
- Gibbs energy predicts whether a process will occur spontaneously at constant temperature and pressure.
- It is defined as, G = H – TS
- ΔG>0; the reaction is non-spontaneous
ΔG<0; the reaction is spontaneous
ΔG=0; the reaction is at equilibrium
- Solubility product is the mathematical product of its dissolved ion concentration to the power of stoichiometric coefficients.